SCADA Supervisory Control and Data Acquisition System
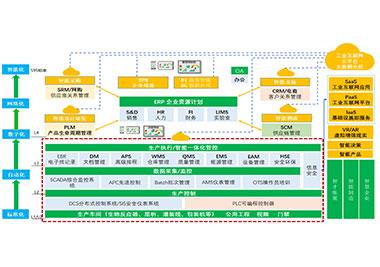
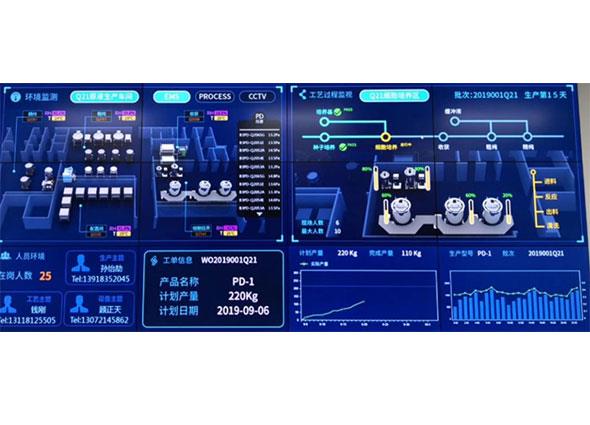
Supervisory Control and Data Acquisition system can realize online monitoring and warning of key data and archiving of production history data by efficient data collection and information storage. It can eliminate automation silos, realize interconnection and interoperability among equipment, and help production managers to centralize the operation of production equipment; it can also provide data basis for data transparency at production line level, workshop level and factory level, ensuring data integrity of production system, and improving the automation and informationization level of production process. For compliance and improving the production efficiency of production-related systems, SCADA system collects key data of on-site production equipment and processes, which will also provide a great convenience and solid data base for the deployment of digital production operation management platform, especially MES, which is a necessary part of the vertical integration of enterprise informationization.
Product Features
-

 The software meets the requirements of FDA 21 CFR Part 11
The software meets the requirements of FDA 21 CFR Part 11 -

 Equipped with complete security measuresIt includes user ID, authorization, password policies, user permissions, shared desktops, remote logins, user profile roles, etc.
Equipped with complete security measuresIt includes user ID, authorization, password policies, user permissions, shared desktops, remote logins, user profile roles, etc. -

 Equipped with comprehensive "permission" mechanismsEquipped with comprehensive "permission" mechanisms. Permissions are assigned when roles are defined, and each user is assigned different permissions which are set to specific user roles (e.g., administrator, supervisor, technician, operator, etc.)
Equipped with comprehensive "permission" mechanismsEquipped with comprehensive "permission" mechanisms. Permissions are assigned when roles are defined, and each user is assigned different permissions which are set to specific user roles (e.g., administrator, supervisor, technician, operator, etc.) -

 Audit optionTo enable audit trails for user actions and logins to suit the requirements of specialized application environments such as FDA
Audit optionTo enable audit trails for user actions and logins to suit the requirements of specialized application environments such as FDA
Product Specification
Material Download
Related Solutions