The implementation of the new policy promotes the development of TCM granule industry in China
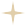
Summary
On February 10, 2021, the State Food and Drug Administration, the State Administration of Traditional Chinese Medicine, the National Health Commission, and the National Medical Insurance Administration jointly issued the "Announcement on Ending the Pilot Work of Traditional Chinese Medicine Granules", which came into force in November. The pilot work of traditional Chinese medicine granules has ended, production and sales restrictions have been relaxed, and the filing of national standards and provincial standards has been accelerated, and China's traditional Chinese medicine granule industry has ushered in new opportunities.
Definition and history of TCM Granules
(一)Definition
Chinese medicine granules are granules made of single-flavor Chinese medicine pieces extracted, separated, concentrated, dried and granulated by water heating, and prepared according to the clinical prescription of Chinese medicine under the guidance of Chinese medicine theory for patients to take [1]. Compared with traditional Chinese medicine pieces, Chinese medicine formula granules have a higher effective utilization rate (70%-80%) and higher gross profit (60%-75%), which has the advantages of no decoction, easy to carry, controllable dosage, convenient to take, stable quality, safe and effective.
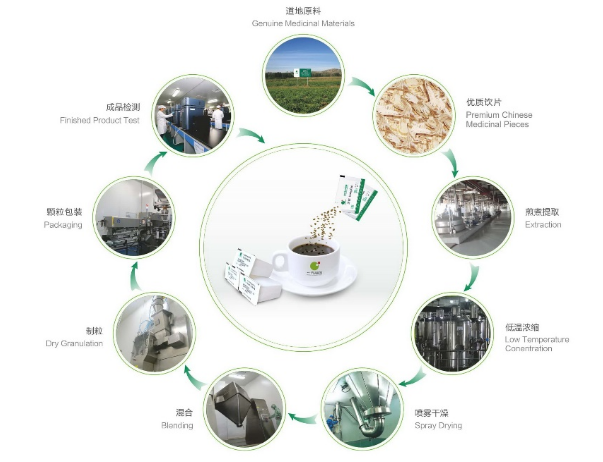 图1:中药Process
图1:中药Process来源:一方制药官网
(二)History
The development history of China's Chinese medicine formulation granule industry roughly goes through three periods, as follows.
1. Research and trial system, exploration period (1977~2000)
The 1977 edition of the Chinese Pharmacopoeia contained a dosage form similar to "scientific Chinese medicine", which became "punch".
In 1987, the Guangdong Institute of Traditional Chinese Medicine, in accordance with the "Opinions on Strengthening the Development of Chinese Medicine Formulations" issued by the Ministry of Health and the State Bureau of Traditional Chinese Medicine, requested the improvement and development of Chinese medicine formula granules, and China's Chinese medicine formula granule industry entered the research and trial stage.
In 1984, Zhang Zhiwei put forward the idea of reforming Chinese medicine tonics, which was the prototype of the later Chinese medicine formula granules.
From 1985 to 1990, during the "Seventh Five-Year Plan", Zhou Yiqun of Jiangxi College of Traditional Chinese Medicine completed a small trial of 101 single-flavored Chinese medicines.
In February 1987, the State Bureau of Traditional Chinese Medicine (SBCM) proposed to conduct research and reform on Chinese medicine tablets.
In 1993, the State Science and Technology Commission and the State Administration of Traditional Chinese Medicine included Chinese medicine formula granules in the "Star and Fire Plan" to further accelerate the development of Chinese medicine formula granules.
In March 1994, the State Administration of Traditional Chinese Medicine approved Guangdong Party and Tianjiang Pharmaceutical as "National Pilot Unit for Reform of Chinese Medicine Tablets", and the research and trial production of Chinese medicine formula granules received the attention of the State Administration of Traditional Chinese Medicine and the support of various national ministries. Through cooperation with scientific research institutes and universities, the Chinese medicine formula granules have made significant progress in process, quality standard, efficacy and clinical comparison research on single decoction and co-decoction.
2. Standardized development, pilot period (2001-2020)
In 2001, the Interim Regulations on the Management of Chinese Medicine Formula Granules were promulgated, formally naming "Chinese medicine formula granules" and stipulating that they should be managed in accordance with Chinese medicine tablets, while approving pilot units for production, and the industry entered a new stage of standardized management.
In 2003, the document "Registration Management Measures (for Trial Implementation)" was drafted to regulate the scientific research, production and clinical application of TCM formula granules in view of the current situation and problems of the use of TCM formula granules.
In September 2012, the Technical Requirements for Research and Development of Quality Standards for Chinese Medicine Formula Granules (Draft for Comments) was released. According to the requirements of this document, by 2015, the pilot producers completed the unification of 681 varieties of process standards.
In 2013, the former FDA stipulated that no self-approval of the production of Chinese medicine formula granules in any name, and only six enterprises in the country could produce them.
In 2015, the former FDA released the pilot production restriction of single-flavored Chinese medicine formula granules, while requiring the implementation of record management.
In August 2016, the State Pharmacopoeia Commission issued the Technical Requirements for Quality Control and Standard Formulation of Chinese Herbal Formula Granules (Draft for Comments), which comprehensively launched the research of national standards for Chinese herbal formula granules, and a total of several enterprises, including the six national pilot enterprises, participated in the research of national standards.
In November 2019, the National Pharmacopoeia Commission issued the "Public Notice on the Pilot Uniform Standards for Chinese Medicine Formula Granules Varieties". Previously, the uniform standards for Chinese medicine formula granules varieties were not clear, which caused multiple troubles for the clinic, and the promulgation of this policy will overcome the above problems and further promote the development of the industry.
3. National promotion, open period (2021 - present)
In January 2021, the FDA issued the Technical Requirements for Quality Control and Standard Formulation of Chinese Medicine Formula Granules to regulate the quality control and standard research of Chinese medicine formula granules and promote the establishment of a unified technical standard system for Chinese medicine formula granules. in February, the Announcement on the End of Pilot Work of Chinese Medicine Formula Granules was issued and implemented since November 2021. in April, the State Pharmacopoeia Commission announced the first batch of 160 National Drug Standards for Traditional Chinese Medicine Formulas, and approved the promulgation of the second batch of 36 National Drug Standards for Traditional Chinese Medicine Formulas in October.
In June 2022, the State Pharmacopoeia Commission published the third batch of 4 national pharmaceutical standards for Chinese medicine formula granules. At present, there are 200 national drug standards in total, and another 69 varieties are in the public announcement period. in August, the National Health Insurance Bureau issued the "Office of the National Health Insurance Bureau on the Issuance of Uniform Coding Rules and Methods for Medicare Chinese Medicine Formula Granules", and the coding rules are divided into 7 parts.
China's TCM Granule industry
(1) Market: rapid development, the market is expected to further expand in the future
Since the new epidemic, the state advocates "insisting on the equal importance of Chinese and Western medicine and the use of Chinese and Western medicine", China's traditional Chinese medicine formula granules are developing rapidly in the post-epidemic era due to the advantages of high utilization rate, high degree of industrialization and convenient transportation and storage. 25.245 billion yuan, an increase of 32.87% year-on-year, accounting for 12.3% of the Chinese medicine tablets. The market is expected to further expand with the full liberalization of the market after the pilot work of Chinese medicine formula granules ends in November 2021.
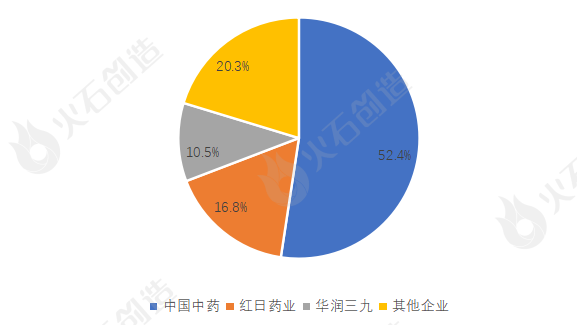
(2) Market competition pattern: Chinese traditional medicine monopolizes half of the country, and the 3+N pattern has been formed
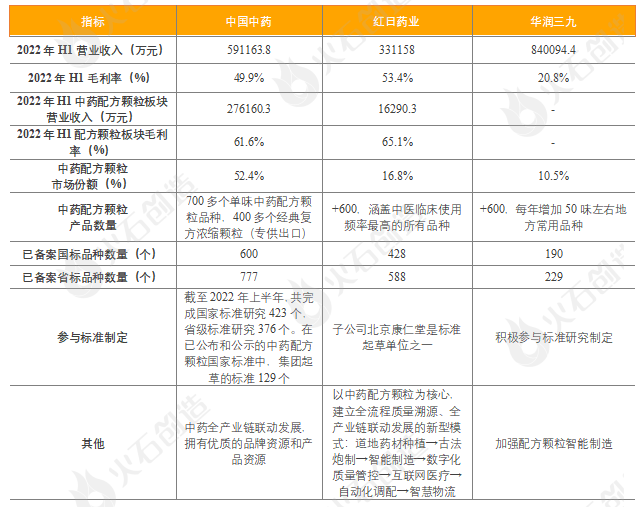
The market concentration of traditional Chinese medicine formula granules in China is very high. According to the statistical publicity of the filing information of the State Food and Drug Administration, as of November 30, 2022, 72 Chinese medicine formula granule manufacturers (including subsidiaries) have filed in China, but most of them have not yet carried out large-scale production and sales. Three state-level pilot enterprises of China Traditional Chinese Medicine, Red Sun Pharmaceutical and China Resources Sanjiu occupy nearly 80% of the market share, forming a "3+N" pattern of Chinese traditional medicine formula granule enterprises. Among them, the first place is Chinese traditional medicine, of which the market share of medicinal formula granules is 52.4%, accounting for half of the country, and is the absolute leader of China's traditional Chinese medicine formula granule industry; The second and third places are Red Sun Pharmaceutical and China Resources Sanjiu, with market shares of 16.8% and 10.5% respectively.
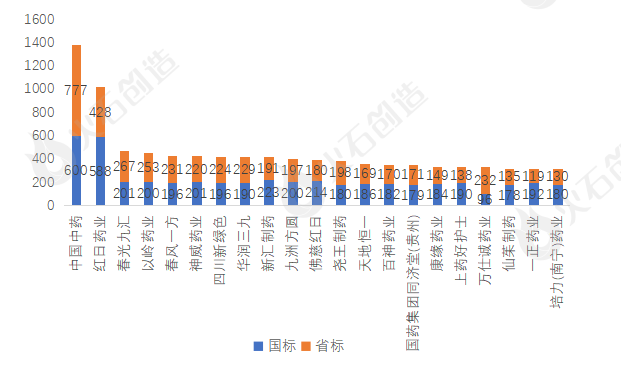
National standard/provincial standard filing, a total of 21 enterprises currently have more than 300 kinds of record-filing varieties. Among them, the number of Chinese traditional Chinese medicine varieties has reached 1377 (600 national standard varieties and 777 provincial standard varieties), of which the filing of existing national standard varieties has basically been completed.
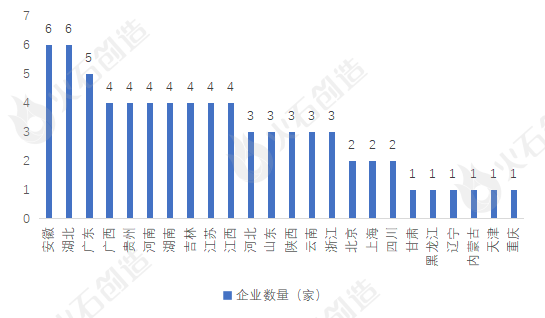
(3) Regional layout: Anhui, Hubei and Guangdong rank in the top three
As of November 30, 2022, among the 72 Chinese medicine formula granule manufacturers (including subsidiaries) that have been recorded, Anhui Province and Hubei Province ranked joint first, with 6 each. The number of traditional Chinese medicine formula granule manufacturers in Guangdong Province is 5, namely Guangdong Yifang Pharmaceutical Co., Ltd., Kangmei Pharmaceutical Co., Ltd., Guangzhou Xiangxue Pharmaceutical Co., Ltd., China Resources Sanjiu Modern Chinese Medicine Pharmaceutical Co., Ltd., and China Resources Sanjiu Pharmaceutical Co., Ltd.
China's TCM Granule industry policy
TCM granules have always been an industry protected by national policies. From 1987, when the state proposed to improve and develop TCM granules, to 1993, the pilot work of TCM granules was launched. In 2001, the granules were included in the management of traditional Chinese medicine decoction pieces. In 2015, it was proposed to formulate a unified drug standard for TCM granules, and then the pilot work of TCM granules was completed in 2021. The market operation has been liberalized and national/provincial standards have been promulgated one after another. The relevant regulatory system and standard system of the TCM granule industry have been continuously improved, and the Chinese TCM granule industry has gradually become standardized and standardized.
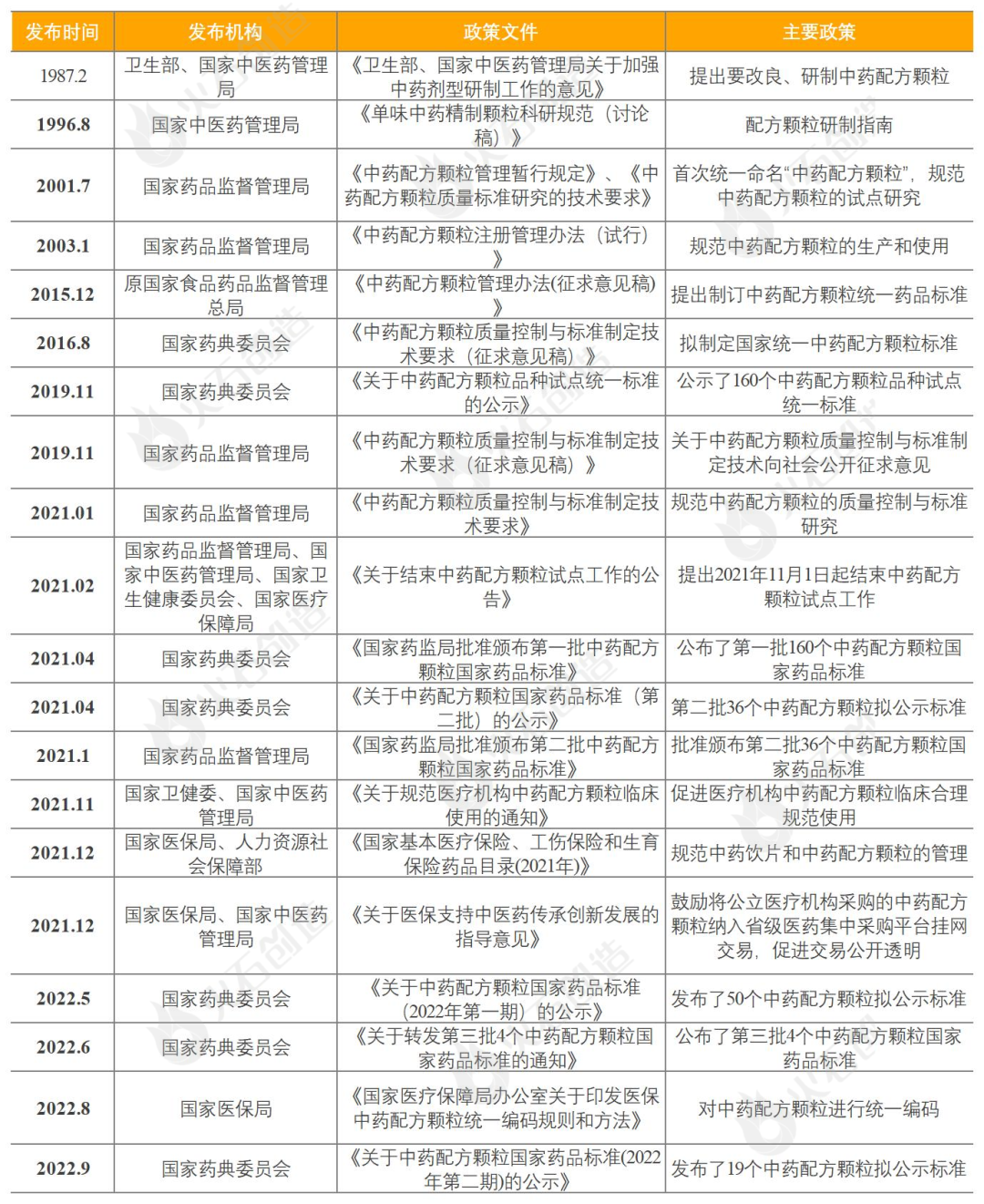
Trends in China's TCM granule industry
(一)With the relaxation of production and sales restrictions, China's traditional Chinese medicine formula granule industry has ushered in new opportunities for development
From Table 3, after the guidance and support of the long-term national policy, China's traditional Chinese medicine formula granule industry was restricted from production qualifications and sales terminals, and by November 1, 2021, the pilot work ended and the filing system was implemented, and the restrictions on production and sales were relaxed, and China's traditional Chinese medicine formula granule industry ushered in new opportunities for development. The following is explained from three aspects: production, sales and medical insurance.
On the production side. The national standard and provincial standard have been steadily advanced, the traditional Chinese medicine formula granule manufacturers have actively carried out the filing of national standard/provincial standard varieties, some enterprises such as the industry leader Chinese traditional Chinese medicine varieties in most regions of the national standard filing work has basically been completed, and non-national pilot enterprises such as Shenwei Pharmaceutical have accelerated the filing, which is conducive to accelerating the industrialization of traditional Chinese medicine formula granules and improving its market penetration.
On the sales side. First, the sales scope of traditional Chinese medicine formula granules has been adjusted from the original secondary and above Chinese medicine hospitals (general hospitals) to medical institutions that can provide traditional Chinese medicine services after approval or filing, and second, manufacturers can carry out cross-provincial sales after meeting the conditions of "filing with the provincial drug administration department of the place of use" or "varieties without national standards should meet the corresponding provincial standards of the place of use". According to the "14th Five-Year Plan for the Development of the Pharmaceutical Industry", in the future, TCM service institutions, especially grassroots hospitals, will expand rapidly, and at the same time, all localities will include formula granules in the treatment rate and into the hospital assessment standards. Enterprises are allowed to sell traditional Chinese medicine formula granules across provinces, and its sales scope has been expanded to medical institutions at all levels, and the scope of use has been greatly expanded, and the market for traditional Chinese medicine formula granules will expand rapidly in the future. In addition, the new standard puts forward higher requirements for the quality and dosage of traditional Chinese medicine formula granules, which promotes a significant increase in production costs, superimposed on the rise in the price of upstream raw materials, prompts enterprises to negotiate price increases for hedging, and increases terminal prices to jointly promote the increase in market scale in terms of sales volume.
On the health insurance side. The "Announcement on Ending the Pilot Work of Chinese Medicine Formula Granules" clarifies that if the varieties of Chinese medicine pieces have been included in the scope of medical insurance payment, the corresponding Chinese medicine formula granules will be included in the scope of medical insurance payment after expert review, and shall be managed with reference to Class B. At present, some provinces and cities include traditional Chinese medicine formula granules in provincial medical insurance, and some provinces and cities treat them as drinking tablets. With the rapid implementation of national standards and provincial standards, and the entry of traditional Chinese medicine formula granules into the provincial platform, more provinces and cities will include traditional Chinese medicine formula granules in provincial medical insurance in the future, and eventually accelerate their inclusion in the national medical insurance system, which is conducive to enhancing terminal payment capacity and further driving the growth of market demand.
Notes.
[1] Source: Technical Requirements for Quality Control and Standard Development of Formula Pellets
[2] The data are as of November 30, 2022. Ltd. (GB/Provincial Standard 204/278), Jiangyin Tianjiang Pharmaceutical Co., Ltd. (GB/Provincial Standard 200/277), Shandong Party Pharmaceutical Co. (GB/provincial standard 198/102), Henan Hongri KangRenTang Pharmaceutical Co.
-END-
Author | Firestone Creation Lily Zhang